The Food and Drug Administration ordered the first enforcement action against a pharmaceutical company for its use of Facebook a month ago, directing drugmaker Novartis to remove a “Facebook Share” widget on the website for its leukemia drug Tasigna.

The widget was encouraging people to share content that misrepresented the drug, the FDA said in its letter, which it publicized last week. The letter also mentioned the “Share This” tool, which the agency said raises similar issues about other social media sites. But the offending text in the Facebook Share widget was drawn from the source’s metadata, content that is written for search engines and usually does not appear on a web page.
From the FDA’s letter:
The shared content is misleading because it makes representations about the efficacy of Tasigna but fails to communicate any risk information associated with the use of this drug. In addition, the shared content inadequately communicates Tasigna’s FDA-approved indication and implies superiority over other products. Thus, the shared content for Tasigna misbrands the drug in violation of the Federal Food, Drug, and Cosmetic Act (the Act) and FDA implementing regulations.
A screenshot showing the widget before Novartis took it down. [via John Mack]
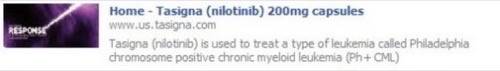
The FDA also dinged Novartis for failure to submit the Facebook Share widget for approval as is required for pharmaceutical promotional materials.
Illegal text was from site metadata
The articles and images in the widget were drawn from online content generated by Novartis. The description of each link was pulled from the meta description in the page’s code, which was also written by Novartis. Normally, that text is less than 200 characters and is visible to users on the results page of a search engine.
Novartis removed the Facebook Share widget after the FDA’s request, but it also made another change. The company edited the description in the metadata of each page to conform to FDA rules.
Metadata not exempt
The FDA letter does not mention metadata. But the same rules that govern advertising, labeling and promotion also apply to metadata, according to a regulatory alert issued by healthcare marketing agency Digitas Health.
And this isn’t the first time the FDA has taken issue with metadata, Digitas Health said – Sanofi-aventis received a letter in March 2009 for metadata on a website for the drug PLAVIX, prescribed to prevent strokes and heart attacks. “The letter received little comment at the time because it was released on the same day as 13 other letters relating to paid search engine marketing. Few people noticed that the infraction cited by DDMAC (the FDA’s Division of Drug Marketing, Advertising, and Communications) related to organic, non-paid search engine results,” Digitas Health said in its regulatory alert.
Metadata violations not uncommon
Social media has been on the FDA’s radar for some time. The agency held so-called Part 15 hearings seeking input on a set of social media guidelines in November last year.
But metadata, which has been appearing in search engine results for a decade, does not seem to have been as high a concern. Some quick searches for popular drugs showed some companies are careful about what statements appear on the search engine result page. But others are not, posting text that could potentially constitute a violation if they appeared in an advertisement or on a website.
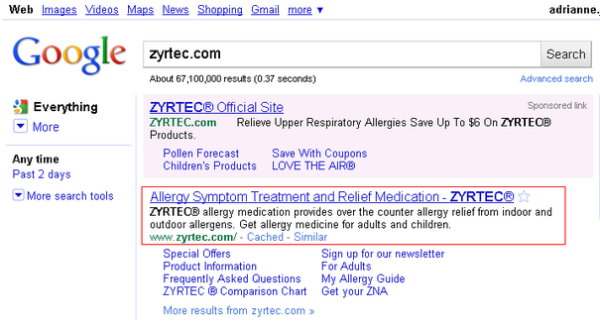
We’re not lawyers. But the FDA cited Tasigna for making “representations about the efficacy” but failing to “communicate any risk information.” This site description would appear to make representations about efficacy by saying Zyrtec “provides relief.”