Researchers at Stanford University have just made a major breakthrough that may impact the technology industry for years to come: they’ve built a better battery. The project, an attempt to use lithium-sulfur in place of the lithium-ion technology that is used in batteries today, has been in development since 2007. Recently, the scientists’ efforts were rewarded when they created a battery that lasts four times as long as its lithium-ion counterparts while also having the benefit of being “significantly safer” than today’s batteries which occasionally explode after short-circuiting.

Although still a ways off from commercial viability (and availability), the lithium-sulfur batteries promise advances like 80% more capacity, 10 times the power density and, theoretically, the ability to last four times as long as modern batteries.
The new battery technology represents the final step in our quest for always-on connectivity to the mobile web. We already have Wi-Fi hotspots, 3G and 4G networks for Internet everywhere and a host of mobile gadgets from netbooks to iPads and mobile phones to notebook computers. But what we haven’t had yet is a way to keep our gadgets powered up for more than a day or so without a charge. That may be soon about to change.
An Always-On Mobile Web
With these sorts of improvements, lithium-sulfur batteries could lead the way in the next phase of the mobile revolution. They could allow us to fully enjoy the web from anywhere in the world, without having to worry about dying batteries, access to power outlets or having to carry around battery replacements when planning long-lasting mobile computing sessions.
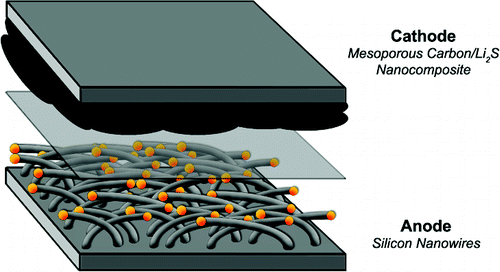
Far more than just a convenience, better battery technology would impact how our mobile devices are designed and how they behave. For example, Apple currently imposes numerous restrictions on members of their mobile device lineup for the sake of battery performance. On Apple iPhones, iPod Touches and the forthcoming iPad, applications aren’t permitted to run in the background and Adobe Flash technology has been banned altogether, supposedly for its CPU usage which rapidly drains battery juice. Other mobile smartphone makers, while not necessarily as restrictive as Apple, still have to weigh the benefits of providing these same types of features with the performance hit their gadgets will take if they do so. And as anyone who regularly fires up their smartphone web browser knows, too much Internet surfing during the day means a phone that dies out before nightfall.
Another example of the technology’s potential impact: e-Readers. Today, if you want to pack your Kindle or Nook device to take with you on vacation, you still have to go through the thought process: how long will I be gone? Will my battery last? Should I pack the cord? Now imagine that you could just throw your e-Reader into your bag without a second thought, just as if you were packing the paperback novel or newspaper these sorts of gadgets aim to replace. Would that encourage more people to make the switch from the analog formats to digital?

The Impacts of Better Batteries
What if, in the future, concerns like these were no longer a worry? What if phones, netbooks, e-Readers and other mobile devices could be used for days on end without the need for a charge? That would radically impact the way we think about and use our mobile devices.
There are a million other use cases that could benefit from this technology change, too, including sensor networks, computing from remote areas, faster news dissemination from areas impacted by disasters (either natural or man-made) where power outages have occurred, gadgets for hikers, campers and other explorers who spend weeks away from civilization and, therefore, away from electricity, mobile location-based services that run in the background on smartphones and other personal mobile gadgets and – OK, we’ll admit it – the ability to Twitter all day long without a recharge.
For the nitty gritty technical details about this new battery technology, MIT’s Technology Review explains everything from the cathodes to the conductivity as well as the challenges still ahead for this breakthrough technology. Most notably, the scientists still need to figure out how to maintain capacity. After five discharge/recharge cycles, the batteries lost one-third of their initial storage capacity and after 40 to 50 cycles, they ceased to function altogether. However, if the researchers can overcome that final hurdle and a few others, the new technology could one day become commercially viable. It’s too soon to know if that will actually occur, but as gadget lovers ourselves, we’re hopeful.




















