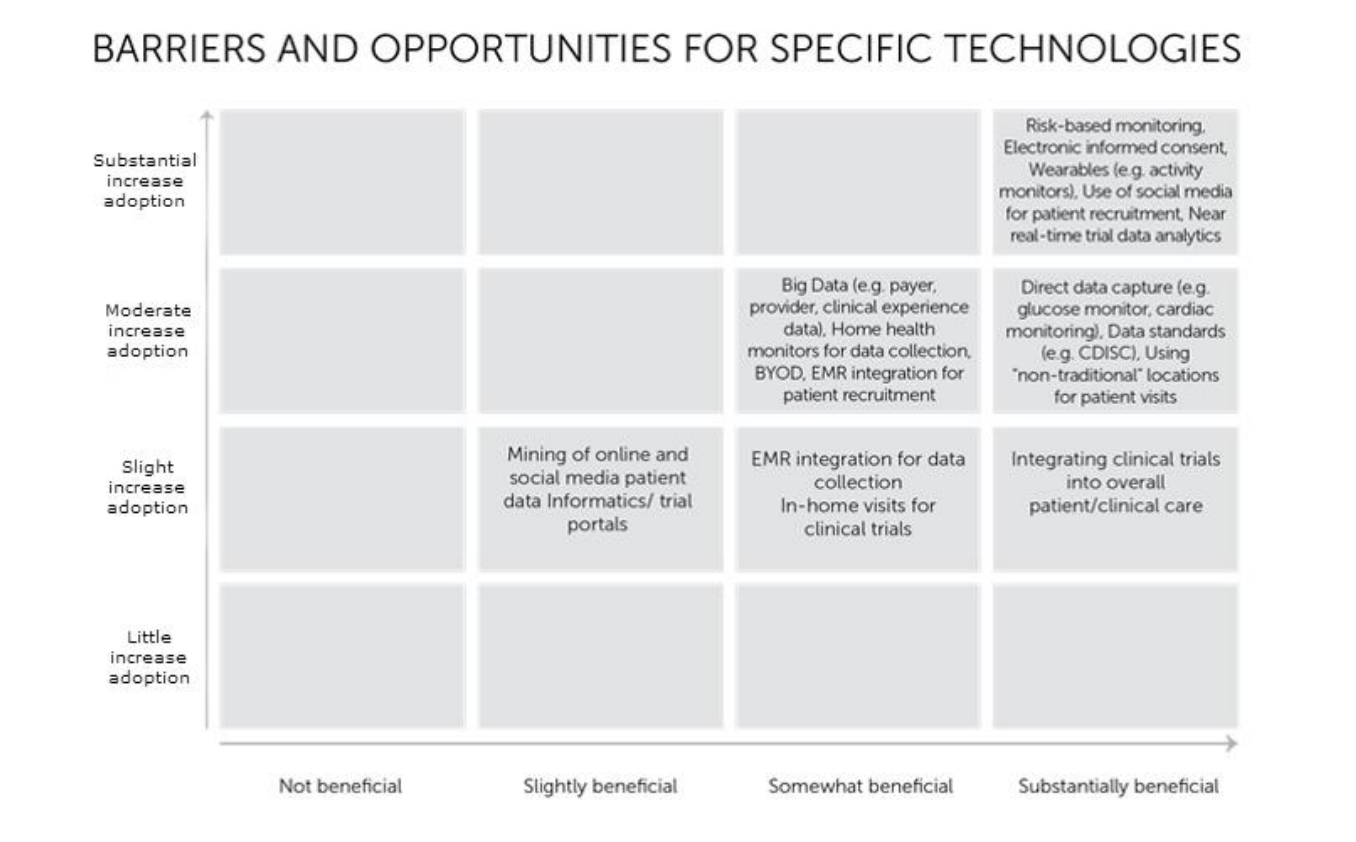
Wearables and social media might dominate the future of medical trials, according to a new survey that asked members of the Association of Clinical Research Organizations (ACRO) what technologies would be most beneficial and see the most adoption in the next two to five years.
The survey results showed five emerging technologies that are substantially beneficial and could see a substantial increase in adoption over the time period: risk-based monitoring, eConsent, wearables, social media, and real-time analytics.
See Also: Will smart pills help remind patients to take their medicine?
ACRO will use the survey results to push the Food & Drug Administration (FDA) to provide further guidance and outline the “do’s and don’ts” for professionals looking to utilize these technologies.
The FDA has already provided guidance and regulation on risk-based monitoring and eConsent.
It hasn’t given a lot of guidance on data capture, monitoring, and privacy for wearables, but consumer wearables like the Apple Watch and Fitbit are underwhelming in the health department supposedly because of FDA obstruction. That might hint that the FDA does not want some wearables tracking health data, though health-focused wearables might avoid this regulation.
Social media also playing a bigger role
Social media is another area where the FDA has been largely absent from the discussion, making medical professionals nervous to converse with patients online and offer medical guidance. A lot of ACRO members don’t seem too invested in social media as a way to track patients — that was noted as one of the least beneficial emerging technologies.
Real-time analysis is already being adopted in medical trials across America, as a way to get more data from patients. ACRO still wants more FDA guidance in this field, to avoid issues with privacy, security, and storage of private information.
It’s clear that while the medical industry moves very slow, professionals are looking at emerging technologies as way to make their jobs easier and help patients gain more benefits outside of the usual checkup. ACRO even mentions in its letter to the FDA that acceleration in this industry would happen much quicker if the FDA was able to give instruction and information on what is allowed and what is not.